What is the FDA’s Role?
In the United States, the FDA is the government agency responsible for reviewing and approving new medicines. Because of FDA’s experience with advanced medical products and its deep scientific expertise, under the BPCIA, the FDA is responsible for implementing the approval framework and for reviewing and approving applications for interchangeable biological products and biosimilar medicines. FDA approval is the gold standard for any medicine sold in the United States.
The FDA has taken significant steps to further develop the biosimilar approval process. The Agency has provided guidance documents on the steps that manufacturers should follow when seeking approval for their new medicines, such as securing meetings with the FDA team and demonstrating biosimilarity, so that biosimilars can be approved and sold in the U.S.
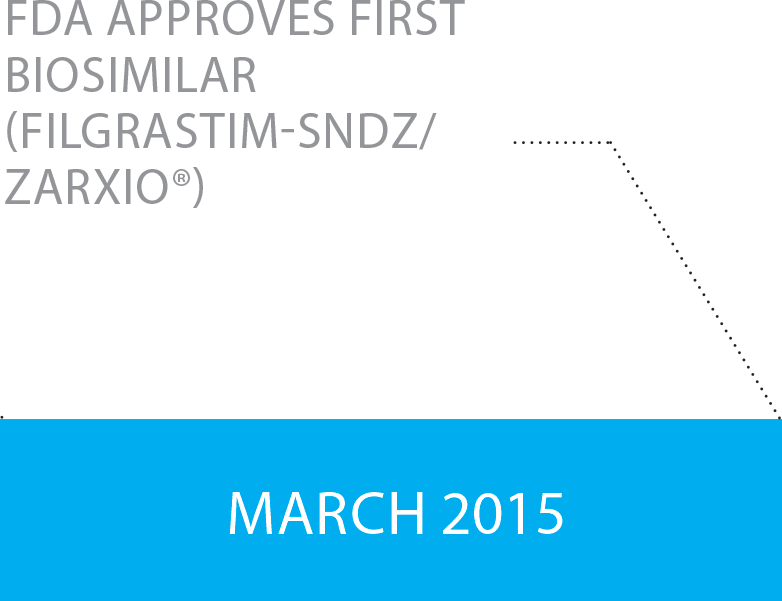
In March of 2015, the FDA approved the first biosimilar medicine, Zarxio® a Sandoz biosimilar for the reference brand Neupogen® approved for each of the five indications sought in its application. Subsequently, eighteen additional biosimilars have been approved in the U.S., and several proposed biosimilars are under FDA review and more than 60 biosimilars are in the pipeline of the FDA’s Biosimilar Product Development Program.