Biosimilar Development Process
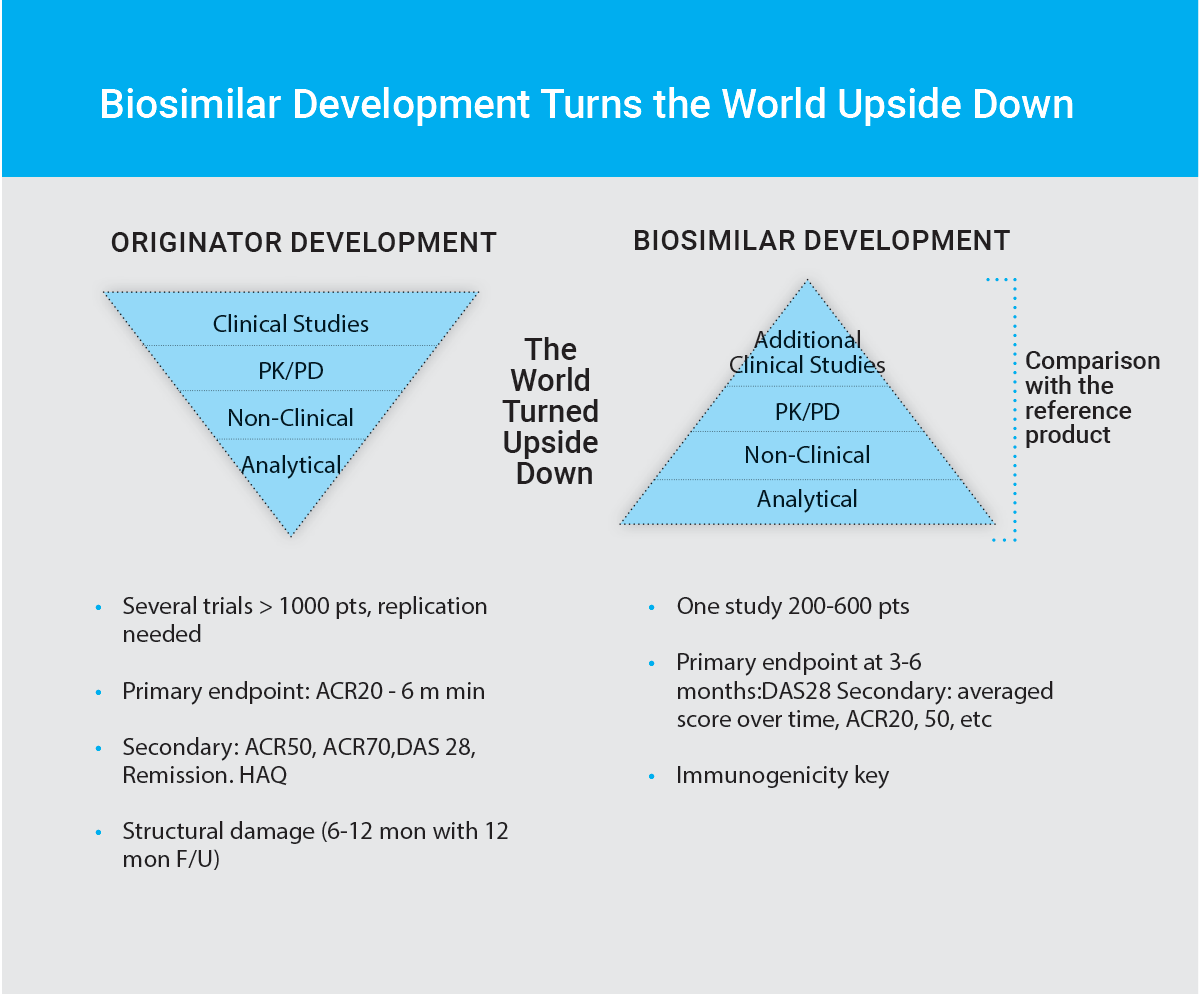
The approach to biosimilar development is fundamentally different to that of new molecule biologics.
New biologics are approved on the basis of demonstration of a significant clinical benefit. This is done by determining efficacy and safety, either against placebo, or currently available standard of care, and this is done through extensive clinical trials.
Typically, clinical trials are conducted in a series of steps, called phases. Each phase is designed to answer a separate research question. Before a drug is approved, there are generally three phases of clinical development:
A new biologic is evaluated in a small group of patients for the first time to look at safety, to begin to determine what is the most appropriate dose for use, and to identify side effects. This is called Phase I. Phase I studies can also include pharmacokinetic (PK) evaluations, designed to determine how the body affects the drug after administration, from absorption to elimination, and pharmacodynamic (PD) evaluations that look at how the drug impacts the body.
The biologic is given to a larger group of patients, further evaluating the best dose, determining if it is effective, and to further evaluate its safety. This is called Phase 2.
The biologic is given to large groups of patients (hundreds or thousands) to confirm its efficacy and further evaluate its safety in studies that are called Phase III. Generally, one phase III study is performed for each product indication sought. As such, the clinical program portion of new biologic development is very extensive, time-consuming and expensive. On the other hand, determination of the new biologic’s molecular structure and function, performed through analytical and in vitro evaluation (analytical characterization), is less intense and comprehensive.