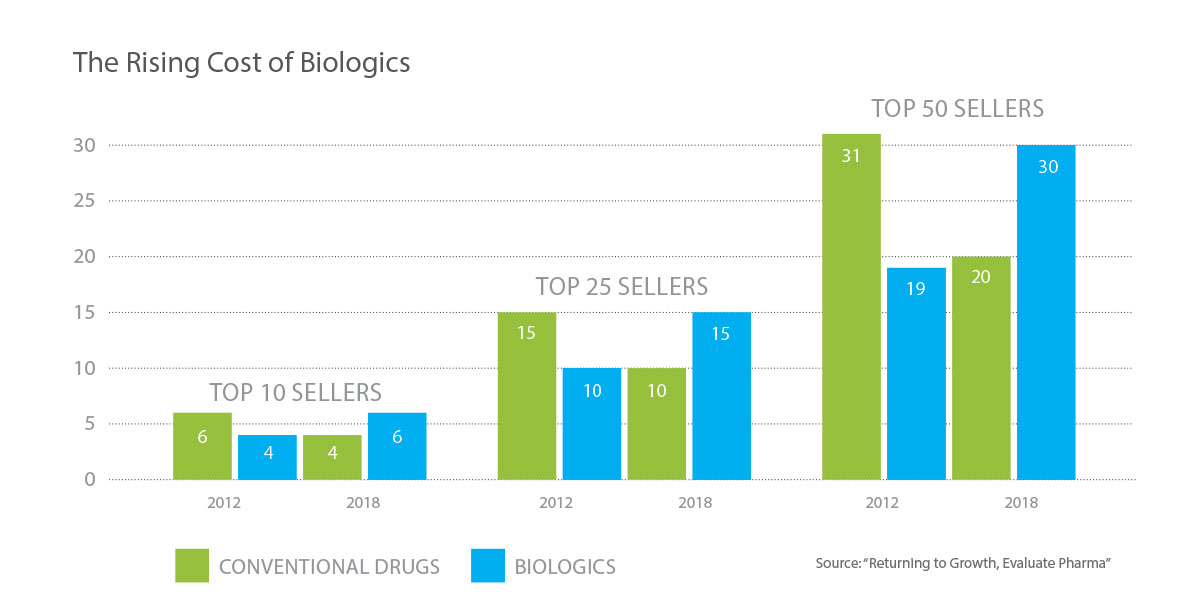
Biologic medicines are costly. While only 2 percent of the U.S. population uses biological drugs, biologics account for 40 percent of prescription drug spending in the U.S. Originator brand biologics can cost the health care system as much as several hundred thousand dollars per patient, per year. Research shows that the average daily cost of a biologic product is approximately 22 times greater than the daily cost for a traditional drug, thus making safe and effective alternatives an imperative for patients. Data also show that biologic prices keep rising. Between 2011 and 2012, prices for specialty drugs—drugs including biologics—increased by 12.9 percent.
Fortunately, more affordable options for many patients who rely on biologic treatments are beginning to enter the market: biosimilars. The approval of biosimilar and interchangeable biologic products will generate competition that lowers costs for patients, providers and the overall health care system.
Some estimates suggest that during the first 10 years of biosimilar availability, consumers could save as much as $250 billion. Biosimilars and interchangeable biologic products are intended to create the market dynamics needed to lower the cost of biologics and provide patients with much-needed access to lifesaving treatments. Furthermore, biosimilars drive competition to treatment categories where there are few options, if any. This competition stimulates further investment and innovation in health care.
The availability of more affordable biologic medicines (biosimilars) translates into enormous savings for patients, taxpayers, insurers, providers, and state and federal governments.